biomarker tracking in clinical trials
Clinical trials of cancer therapeutics, anti-inflammatory biologics, vaccines, antibiotics and cardiovascular treatments can benefit from improved multi-biomarker pharmacodynamic models.
Pharma / BioPharma Clinical Trial Monitoring
tracking protein biomarkers in longitudinal dried blood microsamples
LongitudeDX uses the SISCAPA immuno-affinity MS workflow to accurately measure panels of established and novel protein biomarkers in dried or liquid whole blood or plasma samples. SISCAPA improves the sensitivity and throughput of multiplex MS assays and overcomes the specificity and interference shortcomings of conventional immunoassays.
Combined with the use of dried blood spot (DBS) samples collected using the SampleDiary* (and other methods), the LongitudeDX analytical workflow provides a simple and rigorous approach to track personal biomarker data over time, and enables detection of small health changes from personalized baseline levels.
*Use of the SampleDiary device is currently limited to research and non-diagnostic purposes only. LongitudeDX also makes use of samples collected with a range of other devices including Whatman 903 and Ahlstrom 226 neonatal screening cards, Neoteryx Mitra tips, Tasso and 7th Sense devices, etc.
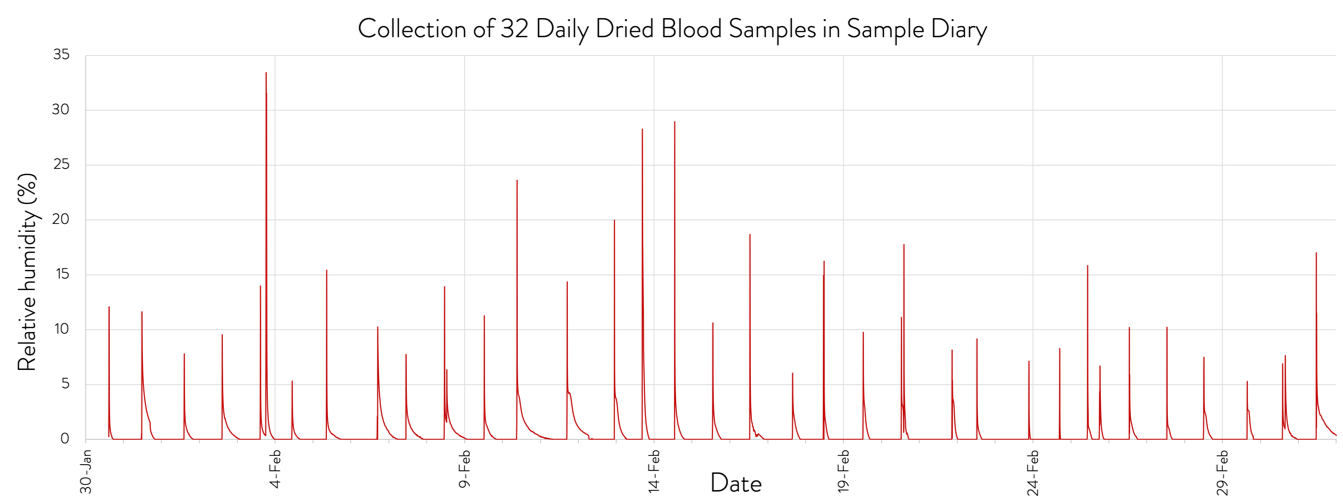
Mobile App Data Collection and Humidity Control
The SampleDiary kit includes access to a simple smartphone app that allows users to accurately record collection of each blood sample. The app uploads sample collection data and makes the record available to study monitors in real time via the TrialKit clinical trial management system. Users of Apple devices can elect to upload Apple Healthkit data as well (e.g., sleep data, heart rate variability, etc.)
The SampleDiary advanced kit includes a temperature (T) and relative humidity (RH) logging device that retains a continuous record of these parameters throughout deployment of the kit. Bluetooth readout of the device from a returned kit provides QC information on sample storage conditions. The RH trace provides an independent verification of the exact time the box is opened and closed, confirming the sample collection time recorded by the app.
An emerging solution for personalized diagnostics
SISCAPA-MS makes it possible to measure numerous clinically important proteins in DBS samples that can be collected periodically by individuals at home (or anywhere) and sent for analysis to a central laboratory by regular mail.
This longitudinal approach provides a new and powerful means of squeezing substantially more clinical value from the biomarkers we have, as well as a superior method for validating and translating novel protein biomarkers to address unmet medical needs in oncology, infection, and neurodegenerative disease.
Benefits of LDX for Clinical Trials
HIGH-RESOLUTION HEALTH TRAJECTORIES
High-frequency sample collection, coupled with high-precision biomarker analysis, generates a high-resolution picture of the trajectory of health change. As always, more measurements yield better models, with less chance of missing critical health events.
Patient access via home-collected dried blood samples
The COVID-19 pandemic has created enormous challenges for clinical trials, reducing access to patients and altering many aspects of participant health. Sample collection by participants at home provides a simple, low cost alternative to conventional blood draws by health professionals, well suited for high-frequency longitudinal sampling to track disease and treatment against personal baselines.
Personalized evaluation of biomarker results
Evaluation of biomarker data against a personal baseline is much more informative than comparison to conventional population reference intervals. The LongitudeDX approach measures ‘normal’ personal baselines, together with ‘normal’ personal standard deviations from that baseline, allowing biomarker data to be presented in terms of statistical significance in the individual. Such an approach significantly increases the power of existing biomarkers to detect significant changes occurring over time.
panels vs. single biomarkers
The increases in statistical power attributable to use of panels (instead of single analyte tests) and personalized test interpretation (instead of conventional population reference intervals) offer a means to extract significant increases in clinical insight from the menu of known clinical protein biomarkers.
Featured Publications
Bioanalysis Editorial
“Squeezing more value from the analytes we have: personal baselines for multiple analytes in serial DBS.”
Bioanalysis Research Article
“Precision multiparameter tracking of inflammation on timescales of hours to years using serial dried blood spots”
Bioanalysis Research Article
“Multiplexed longitudinal measurement of protein biomarkers in DBS using an automated SISCAPA workflow.”